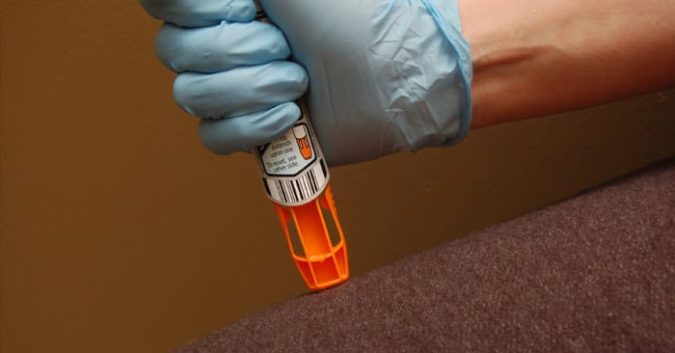
On March 31st, 2017, the U.S. Food and Drug Administration (FDA) alerted American consumers to a voluntary recall from Meridian Medical Technologies affecting 13 lots of Mylan Specialty’s EpiPen® and EpiPen Jr® auto injectors.
Meridan Medical Technologies, a Pfizer company, is the manufacturer of the EpiPen, while Mylan Specialty is the device’s distributor. The voluntary, worldwide recall, which has been expanded to now include the U.S., is allegedly due to potential risk that these devices may contain a defective part that could result in the devices’ failure to activate. Because of this, consumers are at potential life-threatening risk for severe allergic reactions that, upon device failure, could go untreated.
As of right now, the recall pertains to 13 lots of the EpiPen. These lots were distributed to patients between the dates of December 17th, 2015 and July 1st, 2016. They remain the only known U.S. EpiPens to be impacted by the recall at this time.
The Lots Impacted in the United States
| Product/Dosage | NDC Number | Lot Number | Expiration Date |
| EpiPen Jr 2-Pak® Auto-Injectors, 0.15 mg | 49502-501-02 | 5GN767 | April 2017 |
| EpiPen Jr 2-Pak® Auto-Injectors, 0.15 mg | 49502-501-02 | 5GN773 | April 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 5GM631 | April 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 5GM640 | May 2017 |
| EpiPen Jr 2-Pak® Auto-Injectors, 0.15 mg | 49502-501-02 | 6GN215 | September 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 6GM082 | September 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 6GM072 | September 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 6GM081 | September 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 6GM088 | October 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 6GM199 | October 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 6GM091 | October 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 6GM198 | October 2017 |
| EpiPen 2-pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 6GM087 | October 2017 |
If your EpiPen was obtained during this timeframe or has a Lot Number in the chart above, it is highly recommended that you contact both your prescribing doctor and Mylan – the distributor – immediately.
Mylan can be reached by phone at 800-769-9526 and email at [email protected]. Mylan representatives are standing by to answer any customer questions or queries.
The Defective EpiPen® Auto-Injector Device
The EpiPen, which administers an emergency injection of epinephrine is used to treat severe asthma attacks and allergic reactions. It is 1 of the most common emergency medical devices in the world. The device is often thought of by consumers as a “lifeline”; the active drug in Epipens can stop people with severe allergies from going into anaphylaxis, a serious condition in which airways swell. This, of course, can lead to unconsciousness, but also death.
Because EpiPens can be injected immediately after an averse allergic reaction and start working as quickly as 10 to 30 seconds after injection, they are seen by the millions of Americans who use them as “live-saving.” Still, it is recommended by Mylan – and doctors around the country – that because the injection is not a cure and will only stave off anaphylaxis for 5-20 minutes, immediate medical attention be sought in addition to EpiPen use. This advice is stated plainly on the device’s label, and is common knowledge among EpiPen users.
However, with this new wrinkle, and the news that EpiPens might not activate their live-saving medicine at all, the reality of severe allergic reactions, anaphylaxis, and death become even more real for patients everywhere. Consumers should act immediately.
Additionally, the FDA advises healthcare professionals and EpiPen users to report any and all adverse reactions or device malfunctions to the agency’s MedWatch program.