Editorial credit: rblfmr / Shutterstock.com
After the U.S. Food and Drug Administration (FDA) detected asbestos in one lot of Johnson’s Baby Powder®, the company wasted no time in casting doubt over the results.
Johnson & Johnson (J&J) issued its first-ever recall of Johnson's Baby Powder, but it came as part of a statement in which the company laid out possible reasons for a “false positive.” The bottle could have had a broken seal, they reasoned, resulting in contamination. Or perhaps the bottle was a counterfeit. The company promised to work with the FDA in order to “determine the integrity of the tested sample and the validity of the test results.”
Within a few hours, the FDA responded to reaffirm their findings in light of J&J’s statement. The agency stood by the integrity of its testing and results. There was no indication of cross-contamination, an agency spokesperson said, nor was there any record of counterfeit baby powder on the U.S. market.
A week later, on Oct. 29, J&J announced that 15 new tests run by outside labs found that the Baby Powder flagged by the FDA contained no asbestos.
The FDA refused to change its position. Steve Musser, deputy director with the FDA Center for Food Safety and Nutrition, told Reuters, “They (J&J) would say the product is free of asbestos based on their testing, and we would say the opposite for that sample.”
Over the last 7 decades, J&J has managed to successfully bury or undercut valid positive asbestos tests in their talc mines and products.
However, due to damning information that has been uncovered in talc lawsuits, this strategy may no longer work. The recent Baby Powder recall and the ensuing public spat between the company and the FDA show just how much has changed since J&J first discovered asbestos in its talc mines.
Talc Lawsuits Revealed an Asbestos Coverup
Talc is mined all over the world to be used as an ingredient in many commercial and consumer products. Asbestos is often present underground in mineral deposits near talc, and during mining operations, it’s very difficult — some experts say impossible — to make sure that there is no asbestos present in the talc that gets shipped from the mines.
The impurities in freshly mined talc become a problem because exposure to asbestos can lead to mesothelioma and other deadly cancers. Even trace amounts are considered dangerous by the World Health Organization (WHO), which has stated for decades that there is no safe level of exposure to asbestos.
In cosmetics like blush and baby powder, which are used on sensitive areas of the body, the risks of asbestos exposure are elevated. Many people use these talc products multiple times a day for years, starting when they are an infant. Some have filed lawsuits saying their ovarian cancer or mesothelioma is linked to their use of Johnson’s Baby Powder or Shower to Shower.
According to quarterly filings with the Securities and Exchange Commission on Oct. 28, 2019, J&J had 16,800 product liability lawsuits related to body powders containing talc.
The company has always claimed that Johnson’s Baby Powder is free of asbestos and does not cause cancer. For years, they stonewalled efforts of alleged victims who wanted to internal reports about the purity of J&J’s talc, deriding them as “fishing expeditions.”
Yet when the documents were finally made public last year, the simple story J&J had been telling about asbestos-free talc turned out to be untrue. Investigations from Reuters and the New York Times found evidence that the company had privately reckoned with reports of asbestos in its talc dating back to the 1950s.
J&J never told regulators about the positive tests, nor did they choose to warn people who used their talc products every day. Instead, they got together with a cosmetics trade group and lobbied for an acceptable level of asbestos in talc. When that didn’t work, they implemented tests that were not suited for detecting the tiny, lethal asbestos fibers.
Executives at J&J who were responsible for marketing Baby Powder, Shower to Shower, and its other talc-based products had many opportunities to be transparent about the risk of asbestos exposure.
What they chose to do instead was reckless, and totally out of line from the company’s credo, which says their “first responsibility is to the patients, doctors and nurses, to mothers and fathers and all others who use our products and services.”
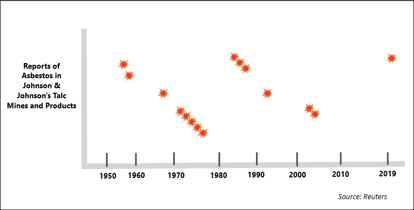
Reading the timeline, which aligns with the internal J&J documents published by Reuters, it’s important to remember that most of this information was kept secret and all of it has been disputed by J&J.
Reports of Asbestos in Johnson & Johnson’s Talc
In the late 1950s, J&J discovered tremolite in one of the mines they used to source talc bound for the U.S. market. Tremolite is one of the 6 types of asbestos known to be carcinogenic.
The others, some of which were later found in Johnson & Johnson’s talc, include:
- Actinolite
- Amosite
- Anthophyllite
- Chrysotile
- Crocidolite
Back in the 1950s, J&J was worried about the abrasive quality of the fibrous, needle-like asbestos minerals in its cosmetic powder. Although there was growing concern about the health risks of asbestos, the company was mainly interested in making sure that their powder remained soft, rather than becoming scratchy due to the presence of asbestos fibers.
In an effort to purify the talc, J&J sent samples to a lab in Ohio. Reports from 1957 and 1958 found that the company’s talc contained trace amounts of tremolite asbestos. This was not just the raw talc taken from their mine — some of the talc that the lab tested was taken directly from the company’s production line in New Jersey.
By 1967, the year tremolite was first discovered in one of J&J’s Vermont talc mines, the dangers of asbestos exposure were starting to be more widely recognized. So were the difficulties of separating talc from asbestos to make a “100% pure” talc-based product.
A 1969 letter from the executive in charge of the company’s talc supply to a company physician admitted it was “normal to find different levels of tremolite in U.S. talc.” The executive wanted to know how much tremolite in their talc products was safe. The physician responded by saying they should limit the amount of tremolite to the absolute minimum. He warned:
“Since the usage of these products is so widespread, and the existence of pulmonary disease is increasing, it is not inconceivable that we could become involved in litigation in which pulmonary fibrosis or other changes might be rightfully or wrongfully attributed to our powder formulations.”
Whether the products did or did not cause lung disease remained a private debate among J&J officials. Even as the dangers of asbestos became extremely clear and publicly known in the 1970s, the company never shared their concerns about asbestos contamination with regulators.
In 1971, researchers at Mt. Sinai Medical Hospital in New York City began testing talc in consumer goods. They were trying to figure out how people who had never worked with asbestos died with traces of the fiber in their lungs. They found asbestos in 2 samples of cosmetic talc, though they never made the brand names public.
Later that year, J&J records show that a mineralogist from Mt. Sinai sent the company a letter informing them he had found asbestos in Johnson’s Baby Powder. Despite its earlier, documented tremolite findings, J&J publicly stated there had never been asbestos in their talc.
The same year, the FDA began an investigation and J&J sent their talc to get tested by private labs. Instead of being transparent about what they discovered, they submitted favorable results to the FDA, but omitted results that showed asbestos in their talc.
The agency never found out about a 1972 finding from a lab at the University of Minnesota that described the presence of “incontrovertible asbestos.” Nor was it made aware of a positive test recorded in 1974 by a Dartmouth professor.
J&J failed to alert authorities even when the results were from their own labs. A 1973 note from a company research director cautioned that no talc product would ever be “totally free” from contamination, and a 1975 report found asbestos in 5 of 17 samples of Baby Powder.
By the 1980s, the dangers of asbestos and mesothelioma were widely recognized, but J&J continued to withhold information about positive asbestos tests from regulators and the public.
Samples taken from its Vermont talc mine in 1984, 1985, and 1986 contained asbestos fibers. In 1990, J&J sold those mines, but in 1992, a report from the new owner identified “clearly visible tremolite and actinolite in specific zones of Vermont deposits.”
For decades, these mines were the main source of talc for Baby Powder, yet the company always claimed their talc was pure. In 2002 and 2003, talc from the Vermont mine, which then produced Baby Powder sold in Canada, found trace amounts chrysotile asbestos. These were deemed “below detection limit,” though there is no amount of asbestos so small as to be assuredly safe.
In the intervening years, J&J’s talc was subjected to more testing, and asbestos was not discovered. The company argued that the negative tests were proof there had never been asbestos in its talc, even though privately, they had known for decades that wasn’t entirely true.
Public Debate About Talc and Asbestos — It’s About Time
With the bombshell Baby Powder asbestos investigations published last year and more than 16,000 talc lawsuits, it was hard to ever imagine that J&J could find itself under more intense scrutiny.
But then the FDA found asbestos in a bottle of Baby Powder.
The discussion about talc safety has been happening behind closed doors for years, but now it is out in the open. J&J can continue to fight the results, but they can no longer claim to be honest with consumers. If they had, the debate about talc and asbestos would have happened decades ago, before so many people were either injured or killed.
All brands are registered trademarks of their respective companies.
